A population of descending neurons that regulates the flight motor of Drosophila
authors: Shigehiro Namiki, Ivo G. Ros, Carmen Morrow, William J. Rowell, Gwyneth M. Card, Wyatt Korff, Michael H. Dickinson
doi: 10.1016/j.cub.2022.01.008
CITATION
Namiki, S., Ros, I. G., Morrow, C., Rowell, W. J., Card, G. M., Korff, W., & Dickinson, M. H. (2022). A population of descending neurons that regulates the flight motor of Drosophila. Current Biology, 32(5), 1189-1196.e6. https://doi.org/10.1016/j.cub.2022.01.008
ABSTRACT
fleeting notes
flies can fly straight after losing half a wing - from this paper
subtle differences in flapping motion require maintenance in flapping motion to maintain stable trajectory.
DNg02 projects from visual output to flight neuropil
there are 15 DNg02 cell pairs
- these neurons regulate wingbeat amplitude over wide dynamic range
- responsive to visual motion during flight
dorsal ventral organization of descending neurons corresponds to motor output.
method:
- used closed loop system - tethered flies
- active stripe fixation
- used 100-ms pulses light - shortest duration that caused changes but minimizes fatigue
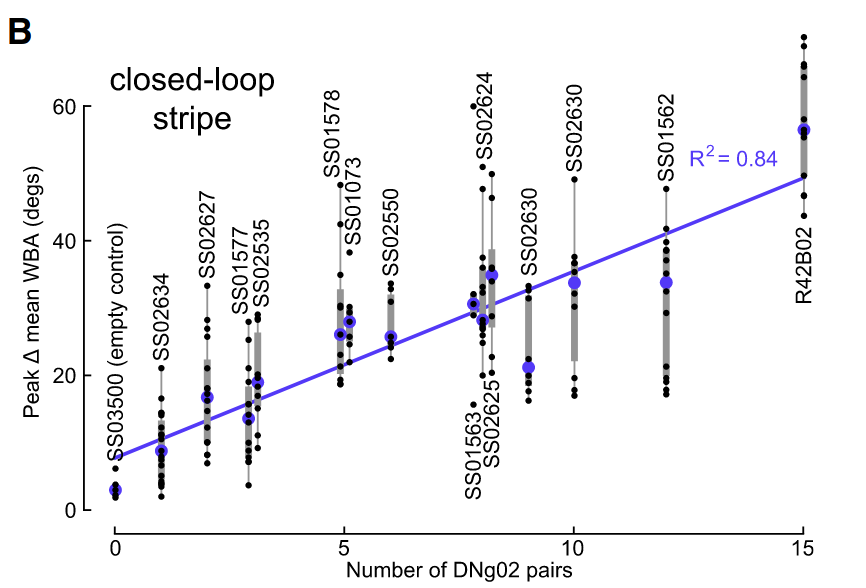
screened driver lines - activating cells and seeing changes in wing beat amplitude
- driver lines labeled varying sets of DNg02 cells. the more cells that were labeled, the larger the change in WBA
DNg02 activity increases wing beat amplitude during flight
noticed that activation changed WBF depending on the baseline WBF of the individual fly and trial.
- WBF stabilized to ~200 hz as the WBA stabilized at ~160 degrees.
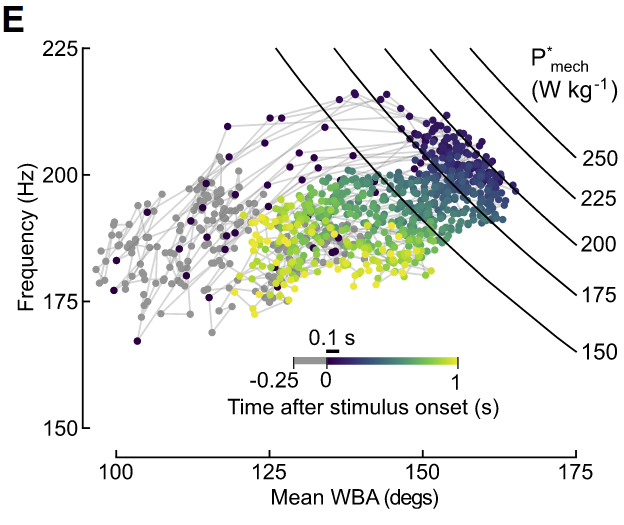
wing kinematics during flight are constrained by a max power output
the mechanical power calculation of the indirect flight muscle
mass specific mechanical power of the flight muscle (P* mech) is the sum of the induced power (P* ind) - the cost of lift - and the profile power (P* pro) - the cost of wing drag -
- this model predicts that when mechanical power produced by asynchronous flight muscles is constant, any increase in WBA must be accompanied by decrease in frequency and vice versa
100ms opto stim resulted in peak activation of the indirect flight muscle because the data in fig 3E are bounded by the approximate force line of 200 W / kg
DNg02 cells can operate independently on each side of the brain symmetrically or asymmetrically depending on the flight maneuver guided by visual information. cell activity is correlated to contralateral wing
highlights
“Drosophila melanogaster is capable of maintaining a stable flight trajectory for periods lasting up to several hours”Page 2
“the neural network responsible for stable flight must be capable of sustaining fine-scaled control over wing motion across a large dynamic range”Page 2
“descending neurons (DNs) that are roughly stratified into a dorsal pathway that projects to flight motor neuropils and a ventral pathway that projects to leg neuromeres”Page 2
“==we observed an even stronger relationship between the change in wing stroke amplitude and the number of DNg02 cell pairs activated (slope = 2.77 per cell pair activated), as well as a higher correlation coefficient (r2 = 0.84)==”Page 4
“Although individual flies varied with respect to their background level of wingbeat amplitude prior to optogenetic activation (Figure 3B, top traces), the peak level of wingbeat amplitude typically reached the same approximate value for each fly during activation”Page 5
“elicited a saturating excitation of the DNg02 neurons within the lines.”Page 5
“That study presented a model in which the mass specific mechanical power (P*mech) delivered by the flight muscles sustains the sum of induced power (the cost of lift) and profile power (the cost of drag) during flight. Profile power, which is the dominant term, is proportional to the product of the wingbeat frequency and amplitude cubed”Page 6
“Thus, this model predicts that when the mechanical power generated by the flight muscles is constant—as it is when the asynchronous flight muscles are maximally activated—any increase in wingbeat amplitude must be accompanied by a decrease in frequency and vice versa”Page 6
“So far, our activation experiments suggest that the population of DNg02 cells might function together to regulate flight power like a throttle, by controlling wingbeat amplitude in a bilateral fashion via symmetric activation of power and steering muscle motor neurons”Page 6
“DNg02 cells can respond differently on the left and right sides of the brain.”Page 6
“wings are novel structures that are not modified from prior ambulatory appendages. Insects retained the six legs of their apterogote ancestors but added two pairs of more dorsally positioned wings”Page 8
“Numerically, however, there appear to be comparable numbers of DNs targeting the wing and leg neuropils.”Page 8
“fact that the control of flight requires greater motion precision because even minute changes in wing motion have large consequences on the resulting aerodynamics.”Page 8
“One means of controlling fine-scaled sensitivity over a large dynamic range is through the use of a population code with range fractionation, a phenomenon that bears similarity to the size principle of spinal motor neuron recruitment.”Page 8